- Introduction
- Principle
- Requirement
- Procedure
- Working instrument component and procedure.
Introduction:-
The spectrophotometer is a routinely used instrument in scientific research spectrophotometer is the quantitative measurement of how much a chemical substance absorbs light by passing a beam of light through a sample using a spectrophotometer.
Principle of spectrophotometer
The Working Principle of photometers and spectrophotometers is based on Beers Lambert’s law same as colorimeter.
Beer’s Law
According to this law the amount of light that passes through the solute concentration and observed light is directly proportional to the concentration of solute.
Lambert’s Law
According to this law A Ray of monochromatic light passes through the absorbing medium it intensity of light decreases as the length of the absorbing surface medium decreases and Increases the absorbing surface medium then the light absorbance maximum increases.
Requirement:-
- 15×25 mm test tube
- 1, 2′ 3, 5, 10 ml Serological pipette.
- Distil Water
- 100 mg/dl pattasium chromate solution.
Procedure:-
- Label the tubes as 10, 30, 40, 60 and 70.
- Pipette distilled water 9.0, 7.0, 6.0, 4.0, and 3.0 ml respectively in the labeled test tubes.
- Pipette dichromate solution 1.0, 3.0, 4.0, 6.0 and 7.0 ml respectively in the above-labeled tubes.
- Mix well and read absorbances at 420 nm (violet filter) or 415 nm (blue filter).
- Plot a graph.
- Evaluate the graph to find out the maximum O.D. on a straight line (limit of the experiment).
Working instrument component and procedure
Component:-
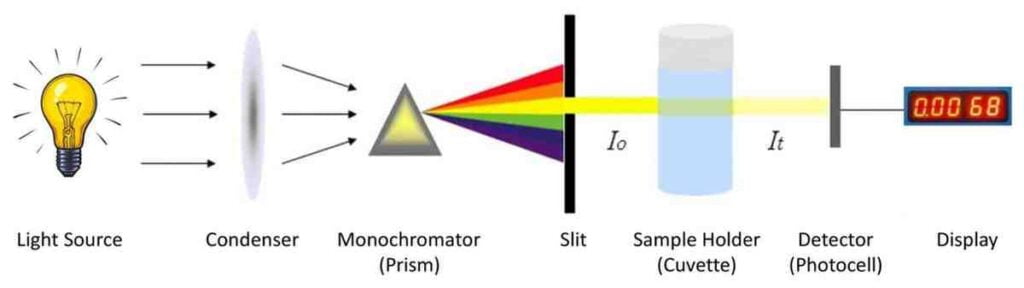
- Light source
- slit
- Condenser lens
- Monochromator
- Sample holder cuvette
- Photo detector
- Display
Light source:-

The light source of the calorimeter produces chromatic light in many directions in the form of light energy.
Slit:-
It allows a beam of light to path and minimize extra light.
Condenser lens:-
Light is condensed by the condenser and on the filter.
Monochromator:-
It is used to produce monochromatic radiation (one wavelength band) from polychromatic radiation (white light) produced from light source. It allows specific wavelength to pass through it. Prism, gelatin fibers, grating monochromators or interference filters can be used.
Sample holder cuvette:-
Color solution present in a cuvette and light is bombarded on cuvette then some light is absorbed and some light is transmitted.
Photodetector:-
A photo meter absorbs transmittance light and changes in electrical form.
Display:-
In clinical laboratory, spectrophotometer is used for the estimation of various biochemical test in variety of biological samples like blood, plasma, serum, CSF, urine and other body fluids. All those methods which involve the formation of colored solution with specific analyte, the analyte can be estimated quantitatively. spectrophotometer are also used in the food industry and textile company etc
Spectrophotometer principle?
Beer’s and Lambert’s law
Spectrophotometer uses in laboratory?
measures the amount of photons (the intensity of light) absorbed after it passes through color sample solution.
What is a spectrophotometer and its uses?
spectrophotometer is an instrument it calculate the absorbance of light by passing of color solution . it is used in laboratory for biochemical test.
THANKU