Benedict’s Qualitative reagent very important for sugar test.
- Introduction of benedict’s qualitative reagent
- Principle
- Requirement
- Procedure
- To confirm the quality of benedict’s reagent
Introduction OF Benedict’s quality reagent
Benedict’s reagent (often called Benedict’s qualitative solution or Benedict’s solution) is a chemical reagent and complex mixture of sodium carbonate, sodium citrate, and copper(II) sulfate pentahydrate. It is often used in place of Fehling’s solution to detect the presence of reducing sugars.
Principle of Preparation of Benedict’s Qualitative reagent:-
The reducing substances such as glucose, vitamin C reduce cupric oxide in the Benedict’s reagent (blue color) to cuprous oxide (green, yellow or red color). When a normal urine specimen is treated with the reagent, there is no change in the color, however, color change is observed when a diabetic patient’s urine (containing glucose) is tested with the reagent.
Requirement
1. Copper sulphate, LR
2. Sodium carbonate, LR
3. Sodium citrate, LR
4. Distilled water
5. Normal urine specimen
6. Diabetic patient’s urine (containing glucose)
7. Hot plate magnetic stirrer
8. Conical flask
Procedure of Preparation of Benedict’s Qualitative reagent:-
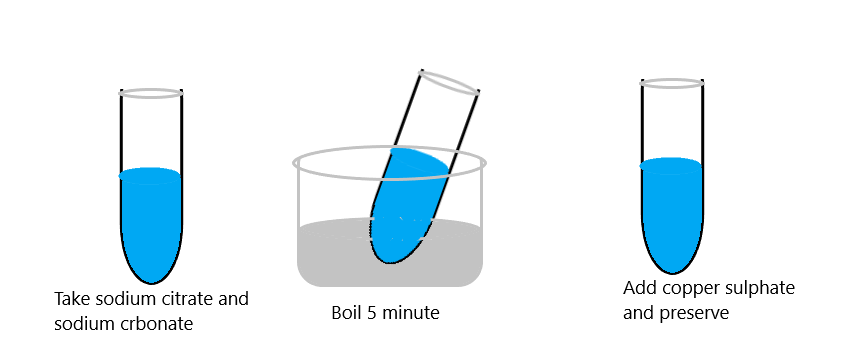
Weigh following chemicals using an analytical balance:
1. Sodium citrate: 17.3 g
2. Sodium carbonate: 10.0 g
3. Put in a conical flask, add 90 ml distilled water, place on a magnetic stirrer and boil for 5 minutes. Cool to room temperature.
4. Weigh copper sulphate: 1.73 g
Add to the above mixture and by introducing the mag- netic stirrer and dissolve copper sulphate completely.
5. By using a measuring cylinder make final volume 100 ml.
6. Store the reagent in a 150 ml polythene container, label appropriately and store at room temperature (25°C ± 5°C)
To confirm the quality of benedict’s reagent
Procedure to check the quality of benedict’s qualitative reagent:-
Test Benedict’s reagent as follows:-
1. Pipette 5.0 ml Benedict’s reagent in two test tubes labeled as N and A.
2. Add 8.0 drops of normal urine by a Pasture pipette to the tube N.
3. Add 8.0 drops of diabetic patient’s urine to the tube A.
4. Keep both the tubes in the boiling water bath for 10 min.
5. Observe the color change.
observation:-
1. No change of the color of the reagent in the tube N is observed.
2. Color of the reagent in the tube ‘A’ changes to green or yellow (or orange to orange red) depending on the concentration of glucose in urine
How to make Benedict’s reagent?
Weigh following chemicals using an analytical balance:
1. Sodium citrate: 17.3 g
2. Sodium carbonate: 10.0 g
3. Put in a conical flask, add 90 ml distilled water, place on a magnetic stirrer and boil for 5 minutes. Cool to room temperature.
4. Weigh copper sulphate: 1.73 g
Add to the above mixture and by introducing the mag- netic stirrer and dissolve copper sulphate completely.
5. By using a measuring cylinder make final volume 100 ml.
6. Store the reagent in a 150 ml polythene container, label appropriately and store at room temperature (25°C ± 5°C)