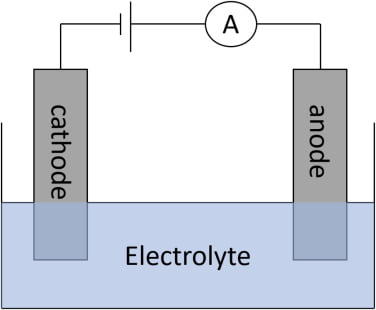
- This technique is used to determine chloride ions in the specimen.
- Most coulometers are based on the principle of constant-current coulometry.
- The coulometer consists of a pair of silver generation electrodes and another pair of silver electrodes to amperometrically detect the end point of the titration.

Table of Contents
Principle of coulometry
It is based on the constant-current coulometry.
Procedure
- The sample is diluted with a mixture consisting of nitric acid, acetic acid, gelatin and thymol blue.
- A timer is activated as soon as the sample is titrated with silver ions generated at the silver anode by the application of a fixed potential of 1 to 2 V.
- Silver ions generated at the anode react with chloride ions in the sample to form insoluble silver chloride.
- Nitric acid provides protons to facilitate the cathode reaction.
- Acetic acid decreases the polarity of the solution and ensures that the silver chloride remains insoluble.
- The gelatin improves the reproducibility of titration.
- Thymol blue prevents microbial growth and serves as a visual indicator.
following reaction
At the anode
Ago—> Ag+ + e–
Ago + cl—>Agcl
At the cathode
2H+ + 2e–—>H2
- The detection of the endpoint of the titration is achieved by the other pair of silver electrodes (to which a constant potential of 0.25 V is applied).
- As soon as all the chloride ions in the sample have reacted with silver ions, the subsequent generation of silver ions results in an increase in the current in the amperometric electrode.
- The increase in current triggers a relay mechanism.
- It stops the timer and the generation of silver by silver generator electrodes.